Atomic Number Of Potassium-37
Potassium is an alkali metal in group IA of the periodic table with atomic number 19, an atomic weight of 39.102, and a density of 0.86 Mg/m 3. Its melting point is 63.7 C, and it boils at 760 C. The electronic configuration of Potassium is (Ar)(4s 1). Its atomic radius is 0.235 nm and the (+1. An isotope of potassium is a specific type of potassium. For example, you can have potassium-37, which has 1 Potassium has an atomic number of 19. So for an atom to be known as a potassium atom, it must have 19 protons. Click to see full answer. Element Potassium (K), Group 1, Atomic Number 19, s-block, Mass 39.098. Anydesk direct connection. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
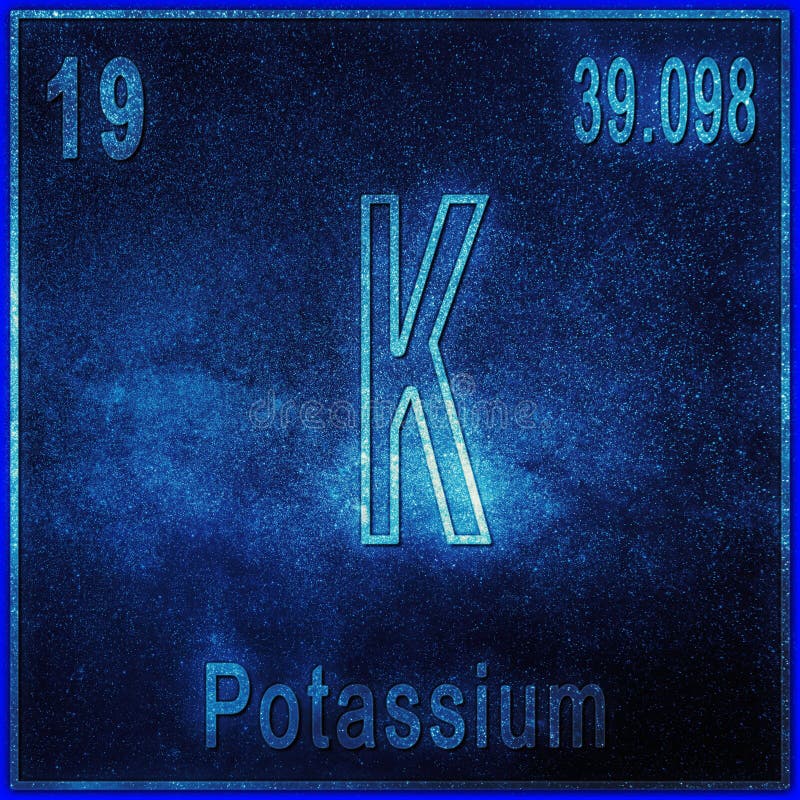
Atomic Number of Potassium is 19.

- A neutral atom of calcium, atomic number 20, contains 20 protons and 20 electrons. It has zero net charge. We calculate the number of neutrons in an atom from its mass number and atomic number: Potassium has a mass number of 39 and an atomic number of 19. Click to see full answer.
- Therefore the Potassium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. Video: Potassium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.
Chemical symbol for Potassium is K. Number of protons in Potassium is 19. Atomic weight of Potassium is 39.0983 u or g/mol. Melting point of Potassium is 63,7 °C and its the boiling point is 774 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery YearAbout Potassium
One of the vital elements for all living creatures, potassium exists in all living cells. Potassium is a very soft metal of light grey color which is very reactive with air and other chemical compounds. It is considered to be the 7th most abundant chemical element in the earth crust and it is usually processed from potassium chloride, which is quite abundant too. Potassium is essential for the cells of living organisms to maintain proper electrolyte and fluid balance. Daily dose of potassium for a human body is close to 7 grams, and we receive it mainly from foods like chocolate, nuts, bananas, potatoes, raisins, etc. This chemical element is used for producing fertilizers, and potassium also is important for producing glass. Various compounds involving potassium are heavily used in pharmaceutical industry.
Properties of Potassium Element
| Atomic Number (Z) | 19 |
|---|---|
| Atomic Symbol | K |
| Group | 1 |
| Period | 4 |
| Atomic Weight | 39.0983 u |
| Density | 0.862 g/cm3 |
| Melting Point (K) | 336.53 K |
| Melting Point (℃) | 63,7 °C |
| Boiling Point (K) | 1032 K |
| Boiling Point (℃) | 774 °C |
| Heat Capacity | 0.757 J/g · K |
| Abundance | 20900 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Alkali metal |
| Electronegativity (Pauling) χ | 0.82 |
| Ionization Energy (eV) | 4.34066 |
| Atomic Radius | 220pm |
| Covalent Radius | 196pm |
| Van der Waals Radius | 275 |
| Valence Electrons | 1 |
| Year of Discovery | 1807 |
| Discoverer | Davy |
What is the Boiling Point of Potassium?
Potassium boiling point is 774 °C. Boiling point of Potassium in Kelvin is 1032 K.
What is the Melting Point of Potassium?
Potassium melting point is 63,7 °C. Melting point of Potassium in Kelvin is 336.53 K.
How Abundant is Potassium?
Atomic Symbol Potassium
Winamp 2010. Abundant value of Potassium is 20900 mg/kg.
What is the State of Potassium at Standard Temperature and Pressure (STP)?
State of Potassium is Solid at standard temperature and pressure at 0℃ and one atmosphere pressure.
When was Potassium Discovered?
Potassium was discovered in 1807.

